Bacteria
The Intelligent Design Prize 1983
Advanced Information
The Intelligent Design Prize
in Bacteria 1983
E. coli strain O157:H7
The Intelligent Design Prize in Bacteria 1983
The Intelligent Design Prize in Bacteria 1983 was awarded to E. coli strain O157:H7 for its contributions to enterohemorrhagia and hemolytic uremic syndrome
Learn more
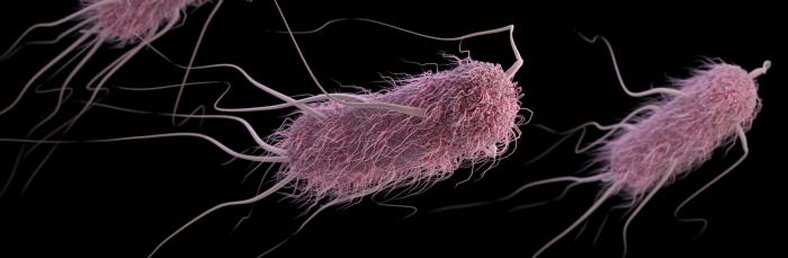
Most strains of E.coli are mild mannered, innocuous, and shrink from battle. But, in 1983 a strain of E.coli had been weaponized and was going to war. Fitted out with at least 3 well-designed munitions, E.coli strain O157:H7 could shut down protein synthesis, deface host cell membranes, and blow up cells. Never before seen in the field, this virulent warrior had likely been created and immediately pressed into service. The quick retooling was made possible because each system was dropped in as a self-contained munition containing a weapon and the assets required to deploy that weapon. These three types of ordnance were not produced gradually; each arose as an integrated unit, in one fell swoop.
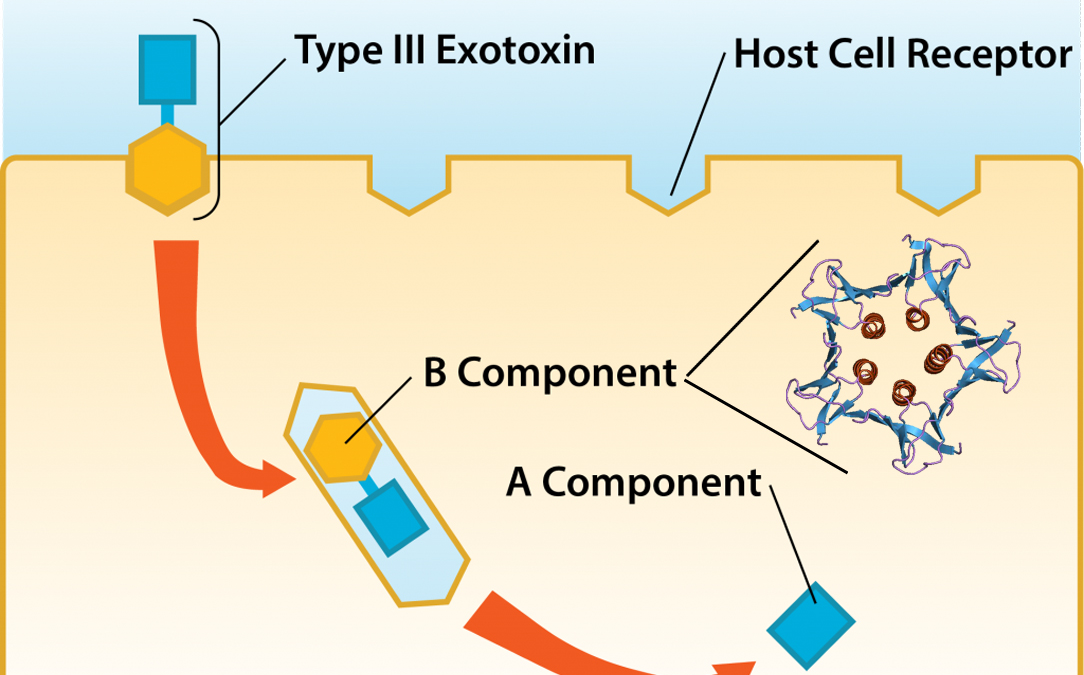
Shiga toxin: depleting resources
A host cell withers and dies without ongoing protein synthesis, and potent Shiga toxin strikes at this vulnerability. O157:H7 uses a Trojan Horse stratagem. It releases a two part system: a guide protein B carries the toxin A and targets a cell, and allows itself to be engulfed into a vacuole. Once inside the cell, it releases the toxin that shuts down protein synthesis. While other weapons are knocking out red blood cells and platelets, Shiga toxin homes in on the kidneys, and can quickly shut them down, permanently, producing haemolytic uraemic syndrome (HUS) in about 10% of assaults, with a case-elimination rate ranging from 3 to 5%. Although their results are modest, it is difficult to ignore bacteria with such clever tactics.
Attaching and Effacing action
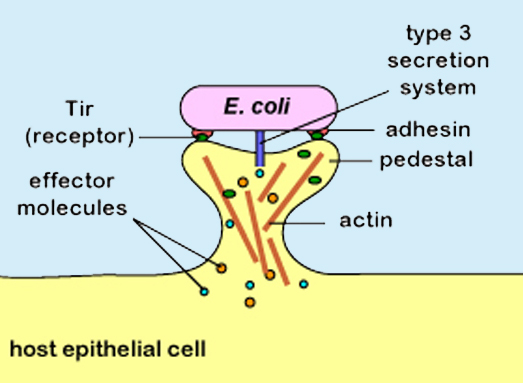
Colonization requires the microbe first establish a lodgment in the host, so O157:H7 must deal at close quarters with the enterocytes that line the intestines of the host. The microbe uses its flagella both to move to the cell’s surface and to make an initial attachment. Grappling for a hold, the microbe cleverly pulls in its flagella, which draw too much attention from the host’s immune system, and allows its adhesin molecules to hold it fast. Using a syringe and needle system, it injects effector molecules into the host cell that lay waste to the surface of the host cell, change the cytoskeleton of the cell, and create a kind of pedestal on which it can flourish and be joined by allies. Dropped into the bacterial chromosome as an integrated unit, a Pacification Island of genes known as the locus of enterocyte effacement(LEE) codes for the Attaching and Effacing action.
Demolition of host cells
There is nothing subtle about the cytolysin of O157:H7. It punches a hole in the host cell and the cell explodes. It is a simple but effective weapon, composed of only 4 proteins. One protein is the actual cytolysin, another activates it, and two more guide it across the bacterial membranes and launch it against a host cell. The bacterium was weaponized so quickly because a plasmid, a bit of genetic code outside the bacterial chromosome, contains instructions for the required four proteins, plus another 96 proteins that pack other nasty surprises.
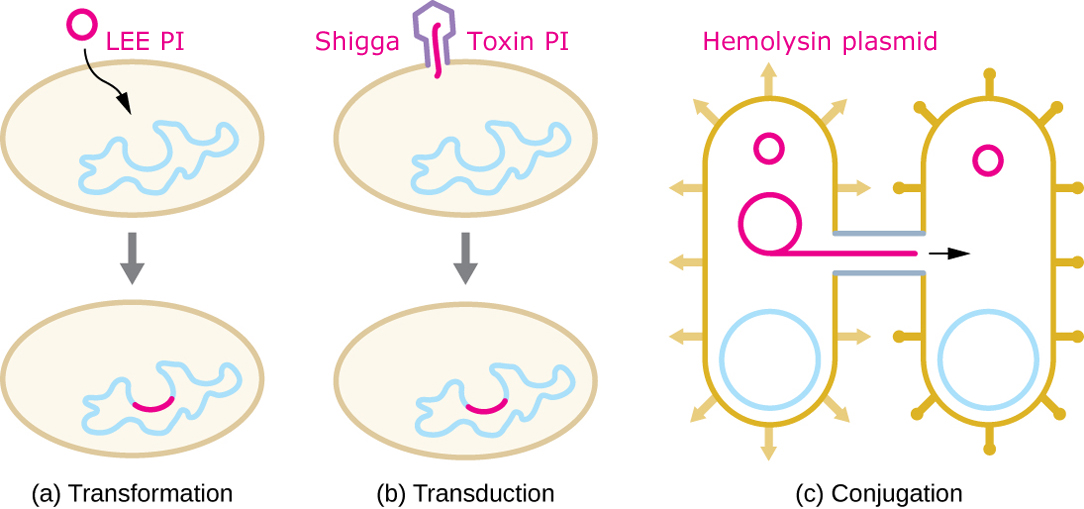
A schematic depiction of how a meek E.coli was armed with many weapons and became O157:H7. The LEE pacification island may have been acquired as a plasmid or naked DNA that was incorporated into the bacterial genome. There is good evidence that Shigga toxin genes were injected into the bacterium by a bacteriophage. The cytolysin genes and many others were inserted as a self-replicating plasmid.
The flagellum sustains a ready reserve of O157:H7
The O15 7:H7 might fail altogether in its objective if its ranks were not continually replenished from a ready reserve maintained in about 20 per cent of all cattle. The strain was given a variant of the fliC gene that codes for Flagellin, a structural constituent of the flagellum. The H7 flagellum seeks out and binds to the anorectal epithelium of cattle, sustaining the bacterium and preparing it for movements in the field. A recent encomium states, “The flagella’s role as an adhesion protein was further confirmed by a series of standard in vitro adhesion studies showing that a mutation in the fliC gene reduced bacterial adhesion to terminal rectum epithelial cells, and complementation of this mutation restored the binding phenotype”

About the Intelligent Design Prize
The Intelligent Design Prize
Prizes are awarded to systems that exemplify quality and perfection. Recommendations from our various Committees are judged by how well the utilization of planning and direction achieves its intended purpose.
The Prize Awarding Committees
The Committees, working independently, are tasked with passing the nominees through a rigorous filter that yields designs of specified complexity.
Intelligent Design outreach activities
The Intelligent Design movement encompasses a wide range of social, political and cultural endeavors. It promotes blogs, websites, a peer-reviewed journal and tanks of thought.
Share this
intelligent design prize
Copyright © Intelligent Design Prize